
Can’t find the answer to your CT lung screening dilemma? Are there conflicting answers to your CT lung screening issue? Do you have a question about CT lung screening that you can’t answer?
Provide a brief description of your situation and get an answer from a team of leading experts in CT lung screening including radiologists, pulmonologists, program navigators, program administrators, thoracic surgeons, oncologists and patient advocates.
Are you an expert? Help the community. Provide your perspective and experience to help address the situations described and answer the questions asked.
Questions & Answers
Questions and Answers
BJM:
Lung-RADS (LR) 4X is a suspicious finding not otherwise defined as LR4A or LR4B.
I use LR4X when something is present other than what is defined in 4A or 4B which is suspicious for lung cancer including:
- Pleural thickening concerning for mesothelial neoplasm
- Enlarged lymph nodes (> 1.5 cm) in the absence of other findings or prior exams to demonstrate stability.
- Findings concerning for lymphangitic neoplasm such as asymmetric interlobular septal thickening.
- Interval stability of a nodule which when compared to more remote exams does meet criteria for growth.
I think point 4 leads to most of the confusion. I will try to clarify below:
If a nodule does not meet criteria for growth (>1.5mm) on several exams over the past 4 years but compared to an exam from 6 years ago it has grown then I will categorize this exam as LR4X and will categorize future exams as LR4X until such time that this nodule either becomes cancer or becomes smaller than the exam from 6 years previous. If a solid nodule is behaving in this way it may be a hamartoma but could also be a carcinoid. If a part-solid nodule is behaving in this way lepidic predominant adenocarcinoma is a concern. Non-solid (pure groundglass) nodules shouldn’t be classified as LR4.
I do not apply this logic to NEW nodules. For a nodule to be NEW there MUST have been a previous CT exam which proves it was not there before. A NEW nodule is much more likely to be either inflammatory or neoplastic than the exact same nodule which has no comparison exam. In other words a NEW nodule is much less likely to be a benign scar that the same nodule for which no comparison exists.
This is why I will report all exams with NEW nodules as LR3 or LR4 depending on the NEW nodule size (exception being nodules highly suggestive of infection or inflammation). If a NEW nodule is stable for more than 3 months I would report the exam as LR2 as this stability is a sign that it may have been an inflammatory process that is turning into a scar. I do not categorize a NEW nodule which is unchanged on follow-up imaging more than 3 months later as LR4X unless there is some other thing going on which makes me think lung cancer is present.
Example Case 1:
- 1/1/2015: Baseline (T0, no comparisons): Several pulmonary micronodules (< 4mm) = LR2
- 1/1/2016: 1st Annual (T1): NEW 6-7 mm nodule right upper lobe (no micronodule on T0 exam in this location) = LR4A
- 4/15/2016: T1 Interval Exam #1 (T1.1): Stable 6-7 mm nodule right upper lobe = LR2 (NOT LR4X)
- 4/15/2017: 2nd Annual (T2): Stable 6-7 mm nodule right upper lobe = LR2
- 4/15/2018: 3rd Annual (T3): 6-7mm nodule right upper lobe grows to 10-11mm = LR4B
Example Case 2:
- 1/1/2015: Baseline (T0, no comparisons): Several pulmonary micronodules (< 4mm) = LR2
- 1/1/2016: 1st Annual (T1): 6-7 mm nodule right upper lobe (previously a micronodule on T0 exam in this location) = LR4A
- 4/15/2016: T1 Interval Exam #1 (T1.1): Stable 6-7 mm nodule right upper lobe = LR4X (NOT LR2)
- 4/15/2017: T1 Interval Exam #2 (T1.2): Stable 6-7 mm nodule right upper lobe = LR4X
- 4/15/2018: T1 Interval Exam #3 (T1.3): 6-7mm nodule right upper lobe grows to 10-11mm = LR4B
- NOTE:
If cancer is detected on this exam it should be ascribed to the T1 round of screening and if yearly cancer detection rate statistics are being compiled it should be counted as a cancer detected as a result of the 2016 (T1 exam).
I have a standard macro for active smokers with ill-defined, centrilobular groundglass opacities which states, “Ill-defined upper lung predominant centrilobular ground glass opacities suggest respiratory bronchiolitis given history of active smoking.” When present I report this in the lung screening specific section of the CTLS reporting template and will make the study a LR2 (assuming no positive findings are present). I instruct the CT technologists to record patient smoking/vaping status at every scan which allows for smoking cessation rate quantification and will enable the CT technologists to direct patient to smoking cessation resources if a patient expresses quit readiness.
Leave a Reply
I classify findings/patterns of nodularity that are specific for infection or inflammation as Lung-RADS® (LR) Category 2 with a subcategory “i” to so I can specifically track the malignancy rate in this group. Even with a multi-step system in place to remind all individuals undergoing CTLS they should be asymptomatic prior to imaging, there is still a fairly steady rate of LR2i findings of about 6-8%. Fortunately, this group of exams has a cancer detection rate of less than 1% in line with what is expected for other LR2 exams and significantly lower than would be expected for LR3 or LR4. However, it is important to ensure that the ordering clinician is aware an infection is possible and to confirm that the findings move to resolution. I recommend consideration of antibiotics and short interval follow-up low dose CT thorax usually in 3 months for an initial LR2i. If the LR2i findings are stable or waxing and waning, as is common for MAI, I may extend the follow-up to 6 or even 12 months as needed. If the 2i finding is more of a consolidation than the more typical multifocal tree-in-bud opacities then I may recommend a followup in 1-2 months or simply make the case an LR 4 and recommend pulmonary consultation to determine the next step.
Leave a Reply
I define Lung-RADS® Category S positive CTLS findings as any unexpected finding which is new or unknown and requires further clinical or imaging evaluation before the next scheduled screening exam. The prevalence of coronary artery calcifications and emphysema is very high in the CTLS population. Over 80% of eligible individuals have some degree of emphysema or coronary artery calcifications and at least 25% of patients have marked emphysema or coronary artery calcifications. Since these findings are expected in this population they are not classified as S positive. I do however report these findings on every report using a four point qualitative descriptors: mild, moderate, and marked. If there are none I don’t mention them in order to keep the CTLS report as simple and focused as possible (no “pertinent negatives”). It is important to note that the rate of clinically significant incidental findings on the prevalence (baseline) exam in the National Lung Screening Trial was 10.2% and about 6% on the incidence (annual follow-up) exams, rates that would not allow for either emphysema or coronary artery calcifications to be consistently included in this group. In clinical practice I have observed prevalence (baseline) rate of 6-7% and 2% on the incidence exams.
Leave a Reply
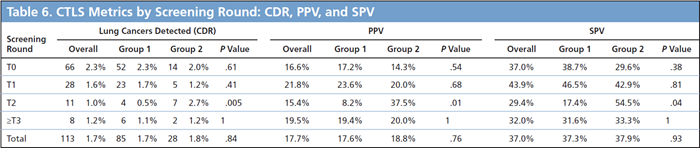
It takes 12 months from the time a CT Lung Screening (CTLS) exam is reported as an Lung-RADS®4 (LR4) to identify 90+% of the cancers that will be eventually diagnosed. As such, in order to assess CTLS cancer detection rates (CDRs) at least 12 months follow-up are required. Shorter follow-up intervals will underestimate the true CTLS CDR. For example, if your program detects 35 cancers detected in 2020 these cancers will be from CTLS exams done in 2020, 2019, and even 2018. Simply dividing the number of cancers detected in 2020 by the number of exams performed in 2020 will over or underestimate the true 2020 CDR.
To accurately assess the CDR for a particular year of screening each baseline or annual follow-up exam performed in that calendar year should be followed for 12 months. This is important not only to assess the CDR but also to account for any false negative exams. Using this approach the CDR for CTLS exams performed during 2019 can be reported in January 2021. It should be noted that even with 12 months of follow-up the calculated CDR will likely underestimate the true CDR by 5-10% as there will be some cases in prolonged imaging follow-up that will only be determined to be cancer after 12 months – see examples below.
When we look at CDR we consider a patient with an LR4 to be suspicious until either they are found to have cancer or are determined via imaging/biopsy to be benign (LR1 or LR2). Separate CDR statistics should be calculated for baseline (prevalence/T0) CTLS exams and annual followup (incidence/T1+) CTLS exams. Incidence rounds of screening (ie T1, T2, T3, T4…) have more similar CDR than the baseline exam and as such for simplicity these rounds of screening can be combined to report an aggregate “incidence screening” CDR. Once the CDR is calculated simply use the same cancer data to calculate the positive predictive value (PPV = CDR/ [LR3 + LR4]) and the suspicious predictive value (SPV = CDR/LR4) .
Examples:
1.) Baseline Screening (T0) 1/2019 (LR2) –> Repeat Annual Screnning (T1) 1/2020 (LR4) –> Cancer detected 4/2020 : DON’T count for 2019 CDR, count as cancer detected in T1 round of screening which was performed in 2020.
2.) Repeat Annual Screening (T2) 1/2019 (LR2) –> Cancer detected 4/2020 (no intervening annual follow-up): Count for 2019 CDR and count as cancer detected at T2 round of screening, but do not count as a false negative as the cancer was detected 12 months after the T2 CTLS exam.
3.) Repeat Annual Screening (T4) 1/2019 (LR4) –> CT follow-up 6/2020 (LR4) –> CT follow-up 2/2021 (LR4) –> Cancer detected 4/2021: Count for 2019 CDR and for cancer detected at the T4 round of screening as there was no intervening LR1/2 exam after the initial LR4 detection in 1/2019.
4.) Interval Scan 1/2019 (LR4) to followup LR3 from repeat annual scan (T2) 7/2018 –> Cancer detected 6/2019: DONT count for 2019 CDR – this cancer is a T2 cancer from 2018.
![]()
Leave a Reply
This is of the often cited barriers to CT lung screening. Many clinicians say it is difficult to have a shared decision making discussion when the average visit time per patient is only 13 minutes. One approach to consider is having group shared decision-making session with several patients eligible for CT lung screening. This approach also allows patients to benefit from each other’s questions, thoughts and experiences.
Leave a Reply
Schedule mammograms and CT lung screening for women at high risk at the same time. Many women who are at risk for lung cancer get annual mammograms but don’t know that they are at risk for lung cancer and don’t know about the option for CT lung screening (CTLS). When discussing breast cancer screening, ask your patient about their smoking history and if they meet the criteria for screening have a shared decision making discussion (SDM) with them. Having the SDM discussion and then scheduling the mammogram and CTLS exam at the same time is likely to increase uptake and adherence to screening.
Leave a Reply
Tell your patients that up until 2015 there has not been a recommended test to find lung cancer early so more than three quarters of lung cancers were found at a late stage when there was a low chance for a cure. The good news is that we now have a screening test for lung cancer that can find it early and the data shows that 80% of the time if lung cancer is found in a screening program it is found at an early stage when the chances for a cure are high. Additionally, there have been new treatments developed for lung cancer that are more effective than what we had even five years ago. With these treatments some people with late stage lung cancer are living for many years.
Leave a Reply
The false positive rate (FPR) for CT lung screeninghas been erroneously reported in many credible medical journals and medical society websites including JAMA Internal Medicine. The reported values are actually the false discovery rate. The FPR in the NLST was 23.5% not 96.4% as many publications mistakenly report. The correct false positive rates for current clinical practice are 7-8%. Breaking it down by screening round, in our program the initial baseline scan (T0) has a FPR of 9.6% and subsequent rounds 5% (T1) and 4.8% (T2). These are better than false positive rates for mammography. For accurate false positive rates please see the following 2 peer reviewed medical journal articles.
Pinsky PF, PhD; Gierada DS, Black W, et al. Performance of Lung-RADS in the National Lung Screening Trial. Ann Intern Med. 2015;162:485-491. doi:10.7326/M14-2086
McKee BJ, Regis S, Borondy-Kitts AK, Hashim JA, French Jr RJ, Wald C, McKee AB. NCCN Guidelines as a model of extended criteria for lung cancer screening. J Natl ComprCancNetw. 2018;16:444-449. doi: 10.6004/jnccn.2018.7021
Leave a Reply
Recommendations are based on each unique exam result. Therefore, if the patient has a follow-up exam as a result of a positive baseline scan, and the result of that follow up exam is negative or benign with a next recommended date in one year, that would be one year from the follow up exam and it would be considered an annual screening exam.

Leave a Reply